Office: Live Oak Hall 1119D | Tel: (818) 677-5212
Lab: Eucalyptys Hall 2008 | Tel: (818) 677-2753
Mail: anna.bezryadina@csun.edu
Research
In the lab we are utilizing optical trapping to manipulate microorganisms, study light-matter interaction, and engineer new generation of biodegradable technology. Light can be used to image tiny objects, cells, and small organisms. Light can be used as medical treatment. Light can be used to understand property of materials. Also, light (laser beams) can be used to trap and move microscopic objects. We control and shape light in time and space, develop novel optical micromanipulation techniques for biophotonics studies, explore how to improve light transmission through scattering media, study effects of ocean microplastics on microorganisms, and engineer new biophotonics systems with light.
Optical Trapping


Optical Tweezers use radiation pressure from a focused laser beam to attract particle to to the the center of the beam (the highest intensity).
Gradient Force - the intensity is greatest at the center of the beam, which pulls the particle towards the center of the beam.
Scattering Force - it is created by light scattering of the surface of the particle, it pushes the particle along in the propagation direction.
For sharply focused beams an equilibrium position can exist slightly past the beam focus at which a particle is trapped. Link to Optical Tweezers Simulation program from University of Colorado Boulder: Simulation program
Optically controlled biofim formation
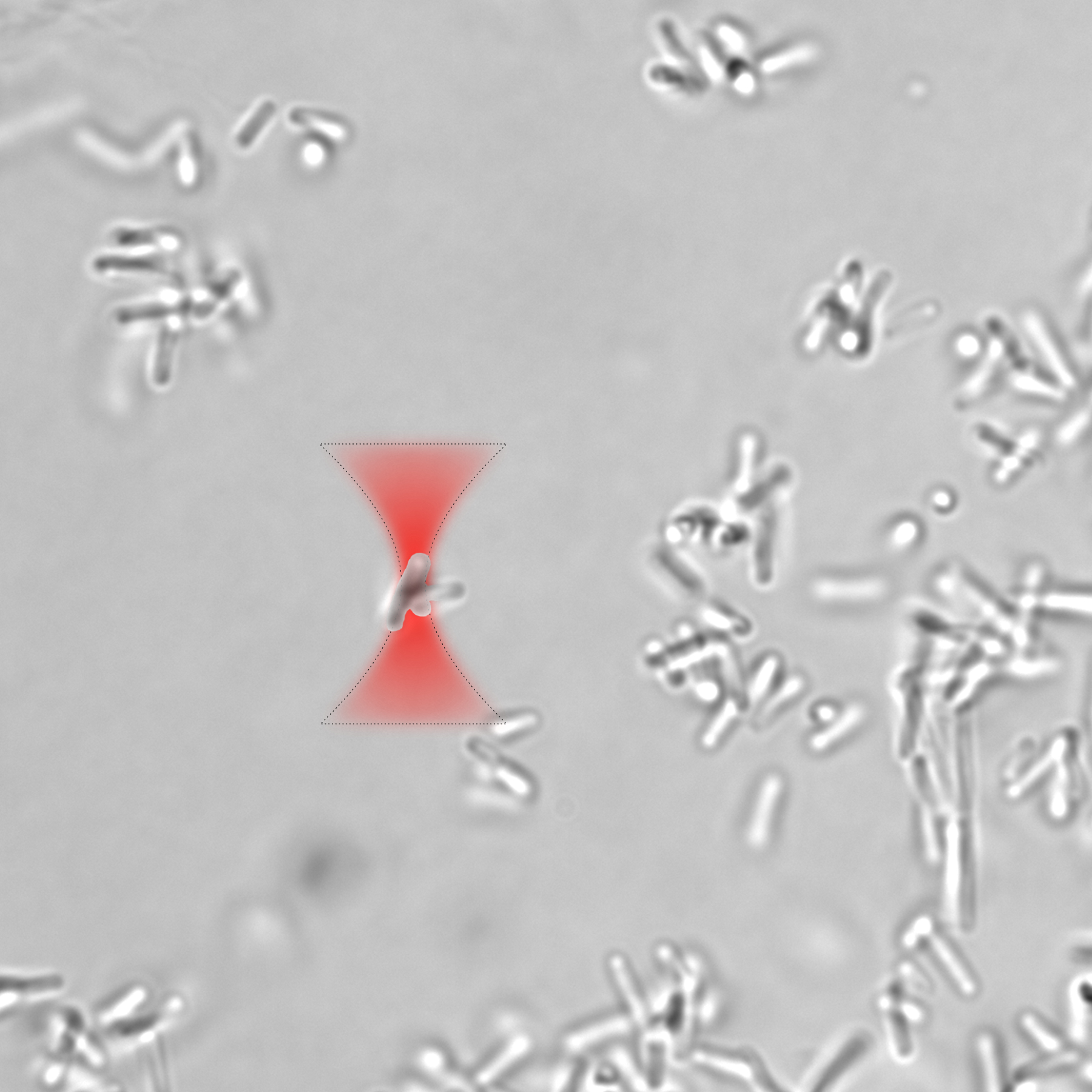

We study how to control and manipulate Bacillus subtilis biofilm growth using laser light.
Biofilm is produced when a bacterias environment becomes hostile and uses biofilm as protection from the environment.
To explore this concept, we study B. Subtilis behaviour in minimal salts glycerol glutamate (MSgg) medium.
Low nutrition environment induces the bacteria to secrete an extracellular polymeric substance (EPS), glue-like substance, and form a biofilm.
In 2024 paper, we investigate the effect of optical trapping on the bacterial aggregation and biofilm development.
Specifically, we determine the most advantageous stage of bacterial biofilm formation for optical manipulation and investigate the
impact of optical trapping at different wavelengths on the aggregation of bacterial cells and the formation of biofilm.
The investigation of optically regulated biofilm formation with optical
tweezers presents innovative methodologies for the stimulation and suppression of biofilm growth
through the application of lasers.
Optical studies of microplastics and nanoplastics and their effect on cells an microorganisms

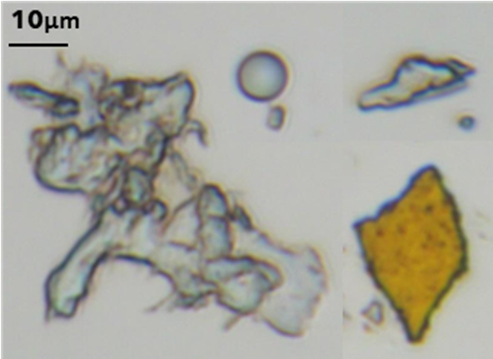
We study opitcal properies of of nature-found ocean microplastics and their potential effects on microorganisms and cells by using an optical tweezers system.
Microplastics and nanoplastics are easy to be taken by microorganisms and then digested up the food chain by the larger organisms and humans.
To collect nature-found microplastics samples,we separate microplastics from water and sand collected at local California beaches.
Due to the irregular shape and varying composition of microplastics, their optical trapping behavior is nontrivial and requires systematic characterization.
To characterize the optical properties of nature-found microplastics, we prepare and analyse lab-made microplastics (PP, PET, and HDPE).
Biological Waveguides

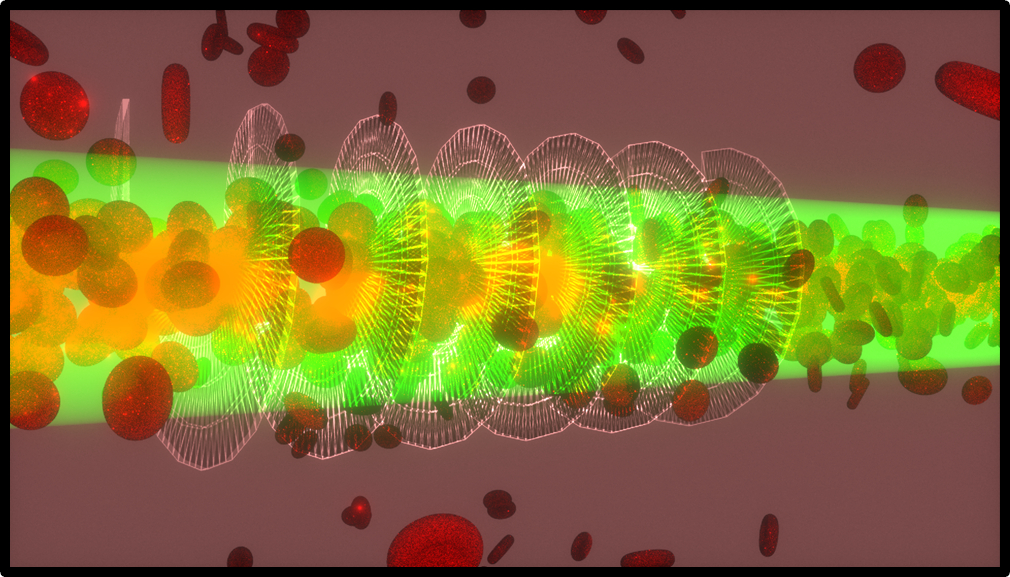
In biological soft-matter environments, such as blood or biological fluids, light typically experiences strong
scattering losses, preventing long distance propagation of light. Such losses limit the development of imaging
technologies and transmissive light applications. In our lab we demonstrated nonlinear self-trapping and the formation of biological waveguides of several
centimeters long without significant photodamage in suspensions of cyanobacteria, E. coli, and red blood cells (RBCs).
Since living cells usually have a slightly higher index of refraction than the surrounding media, suspended microorganisms in biological waveguides get attracted toward
the center of the continuous-wavelaser beam due to the optical gradient force and pushed forward by the forward scattering force.
As a result, hundreds of living cells get trapped along the propagating focused laser beam. The laser beam traps particles near the focus and propels
them forward resulting in selffocusing of the beam due to a cumulative particle lensing effect along the beam path, which allows the formation
of a biological optical fiber or a biological optical conduit.
In 2022 paper, we showed the conservation of photonic states for a range of wavelengths through optically self-arranged
biological waveguides, which can be implemented to transmit light through
scattering media. The conservation of optical properties of light through biological waveguides allows
for the transmission of high bandwidth information with low loss through scattering media.
We experimentally demonstrated the conservation of polarization state and orbital angular momentum of
light through a self-arranged biological waveguide, several centimeters long. Moreover, we use the formed waveguide channels
to couple and guide probe beams without altering the information. The formed biological waveguides are in a sub-diffusive
scattering regime, so the photons information degrades insignificantly over several centimeters of
propagation through the scattering media. Our results show the potential of biological waveguides
as a methodology for the development of novel photonic biosensors, biomedical devices that require
optical wireless communication, and the development of new approaches to noninvasive biomedical imaging.
