Smog
The type of smog we have in
The formation of our smog follows the general daily cycle below:
1. As the day unfolds (Fig. 1), millions of cars find their way onto our roads and freeways as we begin our commutes to school, work, etc.† Itís at this point of the day that auto emissions (CO, NO, and RH) are the highest.† During the morning hours, the air is also very calm because heating by the sun hasnít yet initiated convection and the land/sea breeze cycle.† If an inversion layer is present, it will typically be very low to the ground and pollutants from the previous day may still linger underneath it.
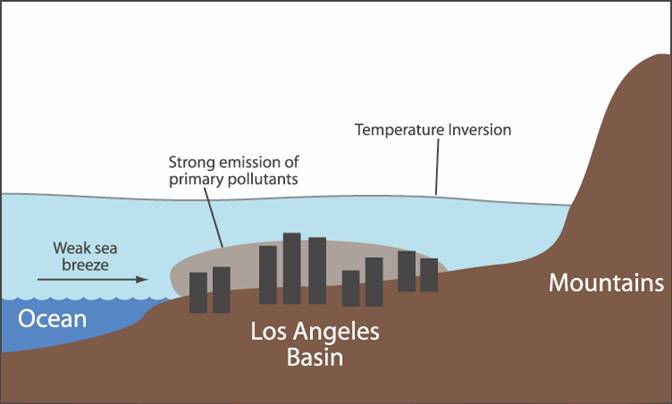 † Fig. 1
† Fig. 1
2. By midday (Fig. 2), we have continuing auto emissions, and the sun has climbed higher into the sky, the photochemical reactions of the primary emission products is triggered as plenty of solar radiation is available.† Now we begin to see higher levels of the secondary pollutants (NO2, O3, and HC), or the familiar brown haze.† Due to increased solar heating, the land/sea breeze cycle also begins around this point in the day, and the pollutants begin to be pushed further inland.
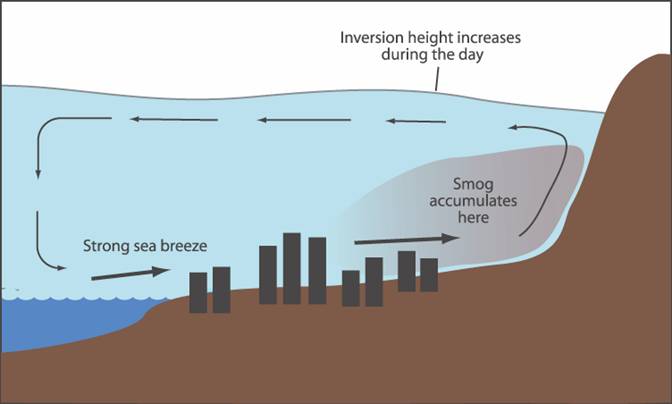 † Fig. 2
† Fig. 2
3. In the late afternoon, O3 concentrations reach their highest levels.† On shore ocean breezes can be quite significant at this point, so smog is pushed far inland.† If the winds are strong enough, the smog can be lifted over the mountains, into the deserts beyond, where it can create a plume of pollution for hundreds of miles.
4. By the early evening, Angelenos are headed home.† So we again see a peak of vehicle emissions.† This second round of primary pollutants is added to the established soup of NO2, O3, and HC.† But because the sun is now much lower in the sky, less solar radiation is present, so photochemical reactions diminish.† The onshore breezes however still continue to push pollutants inland.
5. During
the later evening (Fig. 3), the sea breeze usually diminishes which allows a
land breeze to develop.† This may create
a low level temperature inversion, which traps pollutants below it.† If a larger scale inversion layer is present
during the day,
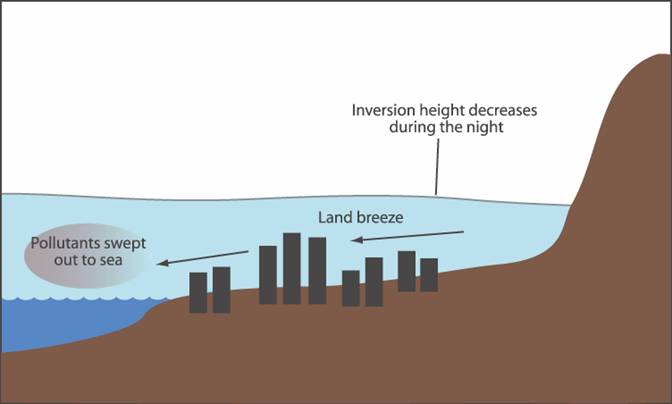 † Fig. 3
† Fig. 3
Written by Anna Huber.
Illustrations from Earth Under Siege, © R. Turco, 2001.
Last updated,