Determination of the mass of carbon dioxide evolved by Alka Seltzer using an electronic analytical balance
SED 695B; Fall 2005
Principles illustrated:
- Conservation of mass
- Diffusion of gases
- Dissolving of gases in liquids

Standards addressed:
California State Standards for Chemistry
3. The conservation of atoms in chemical reactions leads to the principle of conservation of matter and the ability to calculate the mass of products and reactants. As a basis for understanding this concept:
e.
Students know how to calculate the masses of reactants and products in a chemical reaction from the mass of one of the reactants or products and the relevant atomic masses.
4. The kinetic molecular theory describes the motion of atoms and molecules and explains the properties of gases. As a basis for understanding this concept:
b Students know the random motion of molecules explains the diffusion of gases.
6. Solutions are homogenous mixtures of two or more substances. As a basis for understanding this concept:
b.
Students know how to describe the dissolving process at the molecular level by using the concept of random molecular motion
- Balance,
- Water,
- 100mL beaker,
- Thin clear plastic to cover the beaker,
- Alka-Seltzer tablet
As an Alka-Seltzer tablet dissolves in water, it liberates carbon dioxide. The carbon dioxide dissolves in the water and then comes out of solution as a gas.
This carbon dioxide gas has mass, but since it is a gas it escapes from the container and diffuses into the atmosphere.
The loss of mass from the container is measured directly with the analytical balance.
Procedure:
- Refer to the electronic balance users guide to set up the balance. Be sure to level and zero the unit.
- Put about 50 mL of water in the beaker, using the graduations on the beaker.
- Break the tablet into quarters. You need not be accurate.
- Cut a square of the plastic sufficient to cover the beaker loosely, allowing gases to escape but retaining water spray. This keeps the water there to be massed, and prevents spray from damaging the balance.
- Place the beaker and plastic on the balance. Close the door. Do not put the tablet in the balance yet!
- Press the Tare button to zero the balance. This will allow the balance to directly read out the mass of the tablet after it is placed on the balance.
- Open the door, place the Alka-Seltzer on top of the plastic, and close the door.
- Record the mass of the tablet from the display.
- Open the door and allow the quarter tablet to drop into the water. Replace the plastic on the beaker and close the door. The mass displayed will begin to dropas the gas is liberated and dissipated. The display shows the loss of mass of the tablet, which is equal to the mass of the gas liberated. After a time, the display will slow down, but in a 3-hour timeframe it won't stop completely. Tiny bubbles will still be observed in the water as carbon dioxide continues to come out of solution. You have to make a determination of the point at which you wish to consider the experiment completed. It might be helpful to the students to record data at regular time intervals and then to graph the mass lost versus time.




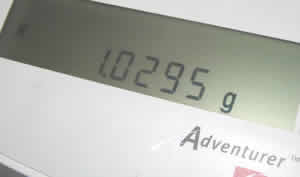


References & Links: