
|
Reverse Osmosis in Water Treatment
To understand reverse osmosis, we first have to understand normal osmosis. And to understand normal osmosis, we have to define diffusion. So here we go...
Diffusion simply says that molecules spread from areas of higher concentration to areas of lower concentration. A simple example may help: if you're boiling a tasty soup in the kitchen, those delicious molecules don't just stay concentrated in the pot -- they gradually move throughout the house, filling the area with the wonderful smells of cooking. In other words, they diffuse from areas of higher concentration (the pot of soup) to lower concentration (the rest of the house).
Osmosis is a special case of diffusion in which all the molecules are in water, and the water crosses a barrier called a semipermeable membrane. A semipermeable membrane allows water to pass right through it, but not ions (e.g., Na+, Ca++, Cl-) and not larger molecules (e.g., hydrocarbons). Semipermeable membranes can be made of many types of materials, but typically are made of cellulose acetate or polyamide resins. An example of a material that is not a semipermeable membrane is Saran wrap, simply because it keeps just about everything sealed in.
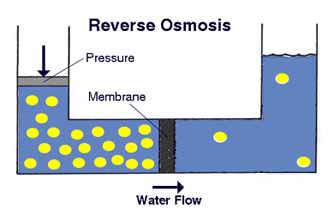
Now here's the twist: in normal osmosis, water moves from areas of lower concentration to higher concentration. That's because the water is acting to dilute or diffuse the higher concentration of other molecules. In the first diagram given below, we see normal osmosis in action. The contaminant molecules are represented by the yellow dots.

|
Normal osmosis doesn't do us much good in water treatment, because the water is moving into a contaminated area. We're interested in reversing the direction. Osmosis can be reversed if sufficient pressure is applied to the membrane from the concentrated side of the membrane. This is shown in the next diagram below. Notice that the semipermeable membrane essentially acts as a filter for the water to pass through, and in fact reverse osmosis is sometimes called "ultrafiltration."
|
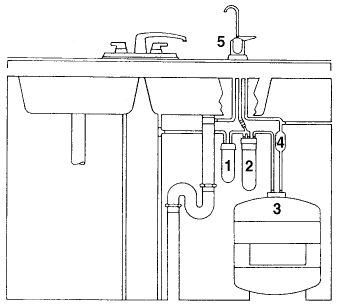
Reverse osmosis (or "RO systems") is a standard in water treatment. The system is normally located beneath the kitchen sink since it is used to treat water for drinking and cooking purposes. A typical RO system is shown in the diagram below.
|
The numbers in the diagram indicate :
If there are any disadvantages to reverse osmosis, they would have to include:
To summarize the main points:
Test your knowledge with a: quiz
For more information, try: water quality