NATURAL SOURCES (also called background levels) in the U.S. add up to about 100 mrems/year. Even without human activities, these sources would still be present. They include the following sub-categories.
1. Terrestrial sources include all land based sources. U.S. community levels range from about 15 to 100 mrem/year. Globally, levels can be as high as 2 rems/year (e.g., parts of Brazil). Various materials contribute radiation, including granite, coal (especially in the western U.S.), and clay (brick homes have typically twice the radiation of wood homes). The most serious radiation exposure from terrestrial sources is usually radon, because it is a gas (i.e., inhalation exposure).
2. Cosmic radiation (also called extra-terrestrial sources) originates from deep space, from such sources as our own sun (a minor contribution) and supernovas. Primary cosmic radiation is mostly protons, some electrons, and various atomic fragments. When these sources hit our atmosphere, their impact creates secondary cosmic radiation, composed of mostly gamma rays, electrons, mesons, and neutrinos. Cosmic radiation varies from 40-160 mrem/year depending on three major factors:
- altitude (e.g., one plane flight cross country can contribute 1 mrem, and Denver has about twice the cosmic radiation of L.A);
- latitude (cosmic radiation is attracted to our magnetic poles); and
- air pressure (as pressure increases, cosmic radiation decreases at ground level).
ARTIFICIAL SOURCES add up to about 80 mrem/year on average.
1. Medical procedures are the major source, with a single chest X-ray contributing an average 200 mrem. Dental x-rays are far less than chest x-rays.
2. Nuclear power plants contribute, on average, about 1 mrem/year. However, this is only for residents closest to the facility. Of course, this does not include other concerns such as nuclear accidents and nuclear waste disposal.
Test your knowledge with a: quiz
For more information, try: radiation
Types of ionizing radiation: Direct (all of the examples in this category are charged particles)
1. alpha particles: two protons and two neutrons (equivalent to Helium atoms stripped of their electrons);
alpha particles have low penetrating power but very strong ionizing power.
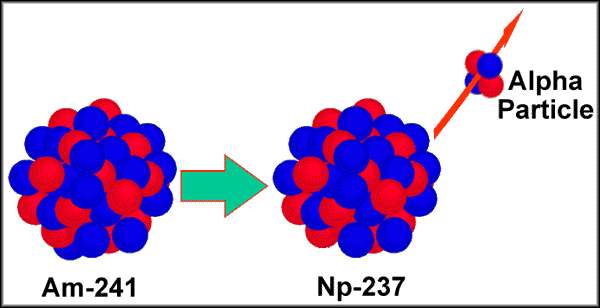
In the diagram below, Americium-241 decays into Neptunium-237 ( a net
loss of 4 in atomic weight) -- the net loss is due to the release of an alpha particle
(made of 2 protons and 2 neutrons).

2. beta particles: electrons travelling at high speed outside of their atomic orbitals
beta particles have moderate penetrating power and moderate ionizing power.
In the diagram below, we see tritium (an isotope of Hydrogen made of 1 proton and
2 neutrons) decaying into Helium (made of 2 protons and 1 neutron). In this
radioactive decay, one of the neutrons has converted to a proton and released a
high speed electon (a neutron contains both a proton and electron).

3. other charged particles: single protons, various charged fragments, etc.
Indirect (these particles are not charged, but they can ionize indirectly)
4. gamma rays: electromagnetic radiation from nucleus;
high penetrating power; weak ionizer
5. X-rays: electromagnetic radiation from electrons;
high penetrating power; weak ionizer
6. neutrons: 1 proton, 1 beta particle, 1 neutrino
Units of Measurement
activity (of source): radioactive decay ("disintegrations")
7. Curie: (Ci) rate from one gm of natural radium-226/second
= 37 billion disintegrations/second
8. Becquerel: (Bq) = 1 disintegration/second
exposure: ionization in air
9. Roentgens: (R) = 1 esu/cc of air = 773,400 esu/kg 10. Exposure unit: 1 coulomb/kg = 3,789 R = 3 billion esu/kg
absorbed dose: energy absorbed
11. RAD: radiation absorbed dose (100 ergs/gram of absorbing material) 12. Gray: (Gy) = 100 RADS
dose equivalent: biological effect
13. REM: roentgen equivalent man = RADs x RBE 14. RBE: relative biological effectiveness (a ratio) 15. LET: linear energy transfer (energy transferred/unit length) 16. Sievert: (Sv) = 100 REMs
Test your knowledge with a: quiz
For more information, try: radiation
Objective: To provide a few examples of the positive applications of our
knowledge of radiation.
1. Iodine-131 tracers: "radioactive cocktail"
A. beta radiation
1/2-life = about 8 days
B. used to study thyroid activity and thyroid treatment
(thyroid concentrates iodine)
note: 99.9% eliminated in about 80 days
2. NAA (neutron activation analysis): "atomic fingerprint"
A. bombard sample material with neutrons
B. measure frequency and intensity of resultant gamma radiation
C. used to measure trace quantities of pollutants
(e.g., heavy metals)
3. Americium-241
A. beta radiation
1/2-life = about 400 years
B. on end of lightning rods, increases attraction
C. also used in smoke detectors:
ionizes air between two electrodes (creates current)
smoke interferes with current, thereby activating alarm
risk of cancer is negligible and outweighed by risk of fires
4. Cobalt-60
A. cheapest form of gamma radiation
B. used in cancer treatments, food irradiation
5. Carbon dating (Carbon-14)
A. how carbon-14 is created in air:
cosmic rays include neutrons:
N + N ---> C + H
in air, C-14 is at stable concentration in CO2
exception -- 20th century activities:
atomic bomb testing
increased CO2 levels
B. how Carbon-14 is used to date materials (e.g., paper)
plants take in CO2 (photosynthesis)
when plants are alive, in equilibrium with environment
upon plant death, C-14 undergoes beta decay (e.g., paper)
1/2-life = 5,760 years
carbon dating accurate up to 50,000 years
Test your knowledge with a: quiz
For more information, try: radiation
1. Non-ionizing radiation: electromagnetic energy that cannot ionize, but may cause health effects
Starting with the highest frequencies:
2. Ultraviolet
exposure: sun, industrial equipment, tanning booths (!)
effects: sunburn, skin cancers
fever, nausea
cataracts, retina damage
controls: clothing, sunglasses, suntan lotions
3. Visible light
exposure: normally no risk
eclipse can cause retinal burn
lasers (light amplification by stimulated
emission of radiation)
effects: burn hole in retina (even at low quantities)
controls: various filters, glasses
4. Infrared
exposure: quickly detected
effects: burns, cataracts, retina damage
controls: special clothing and shielding
5. Microwaves
exposure: radar, T.V., radio, microwave ovens
effects: heating (cooked tissue),
interferes with pacemakers,
association w/ cataracts, cancer, birth defects
controls: distance, materials
6. Electromagnetic fields
exposure: power transmission lines
electric blankets, toasters, hair dryers,
T.V., video display terminals, etc.
effects: not proven !
controls: prudent avoidance (?)
Test your knowledge with a: quiz
For more information, try: radiation