Introduction to Radiation
From the time radiation was first discovered, it has been a term that evokes a certain degree of mystery. Therefore, to really understand various radiation issues, we first need to define the most basic terms.
Radiation = "energy emitted from a source," or "energy that travels."
For example, when you beat a drum, there is a compression of air that occurs upon impact. That air compression travels (radiates) from the drum to your ear, where it may then be perceived as sound. Another example: if you throw a rock into a lake, when the rock hits the water, a circular wave is created that travels (radiates) from the initial impact of the rock. So, radiation is around us all the time. Of course, when you hear of concerns over radiation exposure, it is not because of sound waves or waves in a lake. That brings us to the next definition.
Ionizing radiation = radiation of sufficient energy to ionize atoms and molecules in its path.
An ion is any charged atomic particle (i.e., there is a different # of electrons and protons for the atom). If your body happens to be in the path of ionizing radiation, that ionization may result in health effects (we'll get to the non-ionizing radiation later in this course).
A free radical is any atom with an unpaired electron. Electrons like to occur in pairs, so any unpaired electron is much more likely to be reactive with its surrounding materials. If a free radical is in your body, it will therefore react with all kinds of molecules. Once again, this may exert health effects.
Isotope = any atom with a number of neutrons that is altered from its "normal" state. For example, hydrogen (normally a nucleus of one proton) has two different isotopes by the name of deuterium (a proton plus a neutron) and tritium (a proton plus two neutrons).
A radioactive isotope is an isotope that is relatively unstable and therefore likely to throw off various atomic particles. Once again, if your body is in its path, those particles may exert a health effect.
For all of the above definitions, energy from ionizing radiation is our central concern. That brings us to a set of definitions regarding the four fundamental interactions (or forces) at the atomic level.
1. Gravity = attraction between larger bodies (such as planets and stars).
At the atomic level, gravity is the weakest of all the forces. It cannot adequately explain the interactions at the atomic level. For example, the idea that an atom and its electrons are somehow analogous to the sun and its planets really doesn't hold up, particularly if we are somehow imagining gravitational forces. We need further understanding.
2. Weak forces = forces within certain nuclear particles
The best known example of a weak force is a neutron, which is made up of an electron within a proton. In order to stay intact, it is the weak forces that hold the neutron together. Despite its name, "weak" forces are actually much more more powerful than gravity.
3. Electromagnetic forces = the attraction of opposite charged particles
For example, ionic bonds between negative and positive charged atoms represent electromagnetic forces. This is the second most powerful force at the atomic level, and it helps to explain the bonding of countless chemical bonds.
4. Strong forces = forces that hold a nucleus together
Strongest of all the forces, this is the "awesome force" we refer to when we talk about unleashing the power of the atom, and it is inherent in the energy from nuclear bombs and nuclear power plants. To understand how powerful this force is, consider any atom other than Hydrogen (Hydrogen has only one proton). All the other atoms have more than one proton in the nucleus. But wait a minute! Protons, since they have similar charges, should repel each other. The nucleus should be flying apart if two protons are sitting next to each other, and if that were really the case, the only atom that would ever exist would be hydrogen. Something has to hold these nuclei together, and it happens to be the strong forces.
Now that we have defined the four fundamental forces, we can now define the four fundamental nuclear particles. Of course, there are countless atomic particles that go well beyond the model of neutron - proton - electron. Rather than define and discuss each of these particles, we discuss these four categories.
1. baryons = "heavy particles" that all decay into protons. They include:
- nucleons = found in nucleus (e.g., protons and neutrons)
- hyperons = heavier than neutrons (highly unstable, they decay to neutrons or protons)
2. mesons = "middle particles" found in strong interactions that bind nucleons.
3. leptons = "little particles" that are not subject to strong interactions. The best known example is the electron.
4. photons = particles with zero rest mass (found in light).
Types of ionizing radiation: Direct (all of the examples in this category are charged particles)
1. alpha particles: two protons and two neutrons (equivalent to Helium atoms stripped of their electrons);
alpha particles have low penetrating power but very strong ionizing power.
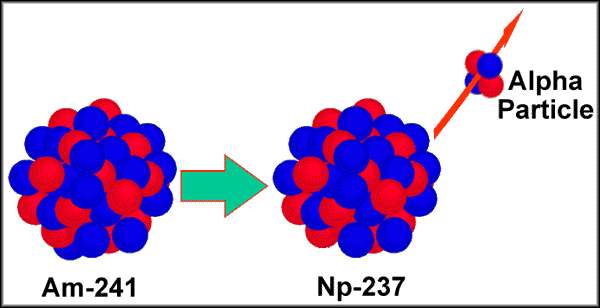
In the diagram below, Americium-241 decays into Neptunium-237 ( a net
loss of 4 in atomic weight) -- the net loss is due to the release of an alpha particle
(made of 2 protons and 2 neutrons).

2. beta particles: electrons travelling at high speed outside of their atomic orbitals
beta particles have moderate penetrating power and moderate ionizing power.
In the diagram below, we see tritium (an isotope of Hydrogen made of 1 proton and
2 neutrons) decaying into Helium (made of 2 protons and 1 neutron). In this
radioactive decay, one of the neutrons has converted to a proton and released a
high speed electon (a neutron contains both a proton and electron).

3. other charged particles: single protons, various charged fragments, etc.
Indirect (these particles are not charged, but they can ionize indirectly)
4. gamma rays: electromagnetic radiation from nucleus;
high penetrating power; weak ionizer
5. X-rays: electromagnetic radiation from electrons;
high penetrating power; weak ionizer
6. neutrons: 1 proton, 1 beta particle, 1 neutrino
Units of Measurement
activity (of source): radioactive decay ("disintegrations")
7. Curie: (Ci) rate from one gm of natural radium-226/second
= 37 billion disintegrations/second
8. Becquerel: (Bq) = 1 disintegration/second
exposure: ionization in air
9. Roentgens: (R) = 1 esu/cc of air = 773,400 esu/kg 10. Exposure unit: 1 coulomb/kg = 3,789 R = 3 billion esu/kg
absorbed dose: energy absorbed
11. RAD: radiation absorbed dose (100 ergs/gram of absorbing material) 12. Gray: (Gy) = 100 RADS
dose equivalent: biological effect
13. REM: roentgen equivalent man = RADs x RBE 14. RBE: relative biological effectiveness (a ratio) 15. LET: linear energy transfer (energy transferred/unit length) 16. Sievert: (Sv) = 100 REMs
NATURAL SOURCES (also called background levels) in the U.S. add up to about 100 mrems/year. Even without human activities, these sources would still be present. They include the following sub-categories.
1. Terrestrial sources include all land based sources. U.S. community levels range from about 15 to 100 mrem/year. Globally, levels can be as high as 2 rems/year (e.g., parts of Brazil). Various materials contribute radiation, including granite, coal (especially in the western U.S.), and clay (brick homes have typically twice the radiation of wood homes). The most serious radiation exposure from terrestrial sources is usually radon, because it is a gas (i.e., inhalation exposure).
2. Cosmic radiation (also called extra-terrestrial sources) originates from deep space, from such sources as our own sun (a minor contribution) and supernovas. Primary cosmic radiation is mostly protons, some electrons, and various atomic fragments. When these sources hit our atmosphere, their impact creates secondary cosmic radiation, composed of mostly gamma rays, electrons, mesons, and neutrinos. Cosmic radiation varies from 40-160 mrem/year depending on three major factors:
- altitude (e.g., one plane flight cross country can contribute 1 mrem, and Denver has about twice the cosmic radiation of L.A);
- latitude (cosmic radiation is attracted to our magnetic poles); and
- air pressure (as pressure increases, cosmic radiation decreases at ground level).
ARTIFICIAL SOURCES add up to about 80 mrem/year on average.
1. Medical procedures are the major source, with a single chest X-ray contributing an average 200 mrem. Dental x-rays are far less than chest x-rays.
2. Nuclear power plants contribute, on average, about 1 mrem/year. However, this is only for residents closest to the facility. Of course, this does not include other concerns such as nuclear accidents and nuclear waste disposal.
1. Non-ionizing radiation: electromagnetic energy that cannot ionize, but may cause health effects
Starting with the highest frequencies:
2. Ultraviolet
exposure: sun, industrial equipment, tanning booths (!)
effects: sunburn, skin cancers
fever, nausea
cataracts, retina damage
controls: clothing, sunglasses, suntan lotions
3. Visible light
exposure: normally no risk
eclipse can cause retinal burn
lasers (light amplification by stimulated
emission of radiation)
effects: burn hole in retina (even at low quantities)
controls: various filters, glasses
4. Infrared
exposure: quickly detected
effects: burns, cataracts, retina damage
controls: special clothing and shielding
5. Microwaves
exposure: radar, T.V., radio, microwave ovens
effects: heating (cooked tissue),
interferes with pacemakers,
association w/ cataracts, cancer, birth defects
controls: distance, materials
6. Electromagnetic fields
exposure: power transmission lines
electric blankets, toasters, hair dryers,
T.V., video display terminals, etc.
effects: not proven !
controls: prudent avoidance (?)
Test your knowledge with a: quiz
For more information, try: radiation
Back to HOME PAGE