
|
Federal Drinking Water Standards
The list of federal drinking water standards is a large and growing one. However, this module presents an initial list that includes standards that have existed for some time. In the early history of the standards, a distinction was made between primary and seconary standards:
Primary standards refer to health based standards that are federally enforceable, usually in the form of an MCL (maximum contaminant level). The enforcement level in these standards is reflected in the legal language of "shall not exceed MCL."
Secondary standards refers to more aesthetically related guidelines given to the states (i.e., are not federally enforceable). The enforcement level in these standards is reflected in the legal language of "should not exceed MCL."
We finish this module with a list of primary standards. With the clear warning that this list is not complete and the effects are not discussed in detail, this nevertheless starts an initial review of the range of health effects from contaminants in drinking water.
|
|
|
arsenic (As): GI tract cadmium (Cd): kidneys chromium (Cr): liver, kidneys |
selenium (Se): neurotoxin lead (Pb): anemia, kidneys, CNS mercury (Hg): inorganic form: kidneys.
|
|
barium (Ba): muscle stimulant flourides (Fl): dental mottling nitrates (NO3-): methemoglobinemia silver (Ag) argyrosis (discolors skin,mucous membranes, whites of eyes) |
|
|
|
|
1,1 dichloroethylene: liver and kidneys p-dichlorobenzene: liver and kidneys 1,1,1 trichloroethane: liver, circulatory, nervous system |
endrin: lindane methoxychlor |
|
2,4,5-T cancer; 2,4-D; vinyl chloride; benzene; trichloroethylene; carbon tetrachloride; 1,2 dichloroethane; trihalomethanes |
|
The Safe Drinking Water Act (SDWA)
PRE-SDWA:
The U.S. Public Health Service originally issued standards for drinking water. They developed the primary and secondary standards that were covered in our last lecture.
SDWA:
1974 saw the passage of the Safe Drinking Water Act (SDWA). Several things were fundamentally different about this law:
The primary federal agency that oversees water quality is the Environmental Protection Agency. However, a number of federal agencies are involved with various aspects of water quantity and quality, including:
Progress in adding standards was slow, however, as scientists in 1974 were just beginning to detect many of the synthetic organics that find there way into water.
1986 AMENDMENTS:
Congress gradually became disenchanted with the EPA's progress, which led to the 1986 SDWA Amendments. This law required addition of 83 contaminants by 1989, and required adding 25 contaminants to the list every 3 years thereafter. Examples of new standards from this law include various VOCs in water, and inorganics such as aluminum, nickel, and beryllium. At the same time, the new law designated "best available technologies" for water treatment.
1996 AMENDMENTS:
The 1996 SDWA amendments canceled the schedule of 25 new standards every three years, and in its place set up a mechanism to set standards based on the occurrence and assessed risks of contaminants. The law also authorized $1 billion in federal grants to individual states for upgrading water treatment systems. Research programs were set up for a consortium of American and Mexican Universities (especially for states on the Mexican border), for estrogen screening programs, and for general research.
Reverse Osmosis in Water Treatment
To understand reverse osmosis, we first have to understand normal osmosis. And to understand normal osmosis, we have to define diffusion. So here we go...
Diffusion simply says that molecules spread from areas of higher concentration to areas of lower concentration. A simple example may help: if you're boiling a tasty soup in the kitchen, those delicious molecules don't just stay concentrated in the pot -- they gradually move throughout the house, filling the area with the wonderful smells of cooking. In other words, they diffuse from areas of higher concentration (the pot of soup) to lower concentration (the rest of the house).
Osmosis is a special case of diffusion in which all the molecules are in water, and the water crosses a barrier called a semipermeable membrane. A semipermeable membrane allows water to pass right through it, but not ions (e.g., Na+, Ca++, Cl-) and not larger molecules (e.g., hydrocarbons). Semipermeable membranes can be made of many types of materials, but typically are made of cellulose acetate or polyamide resins. An example of a material that is not a semipermeable membrane is Saran wrap, simply because it keeps just about everything sealed in.
Now here's the twist: in normal osmosis, water moves from areas of lower concentration to higher concentration. That's because the water is acting to dilute or diffuse the higher concentration of other molecules. In the first diagram given below, we see normal osmosis in action. The contaminant molecules are represented by the yellow dots.

|
Normal osmosis doesn't do us much good in water treatment, because the water is moving into a contaminated area. We're interested in reversing the direction. Osmosis can be reversed if sufficient pressure is applied to the membrane from the concentrated side of the membrane. This is shown in the next diagram below. Notice that the semipermeable membrane essentially acts as a filter for the water to pass through, and in fact reverse osmosis is sometimes called "ultrafiltration."
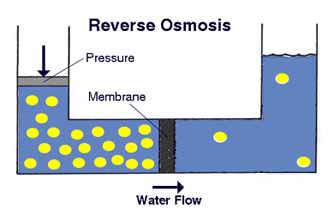
|
Reverse osmosis (or "RO systems") is a standard in water treatment. The system is normally located beneath the kitchen sink since it is used to treat water for drinking and cooking purposes. A typical RO system is shown in the diagram below.
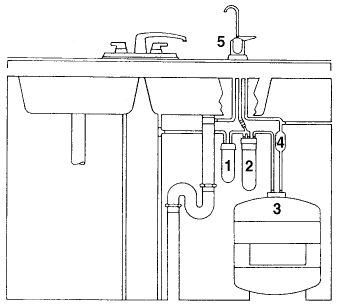
|
The numbers in the diagram indicate :
If there are any disadvantages to reverse osmosis, they would have to include:
To summarize the main points:
Objective: The purpose of this assignment is:
Hard water is water that contains hardness minerals (e.g., calcium, manganese and magnesium) above 1 GPG (grain per gallon). 1 GPG is equal to 17.1 PPM of water hardness as defined by Standard Methods. Hard water is not as efficient or convenient as "soft water" for bathing, washing (dishes, clothes or cars), shaving, and many other uses. For example:
If the hardness is over 3 GPG, softening can usually save enough to pay for the cost and maintenance of a water softener. Water softeners can also remove copper, iron, and other minerals. De-ionization is a more extensive form of water softening that removes anions as well as cations.
Test your knowledge with a: quiz
For more information, try: water quality
Back to HOME PAGE